
The torr is useful for measuring very small pressures, like in a vacuum chamber. In terms of the atmospheric pressure, 1 atm = 760 Torr (exactly). The unit torr is almost equal to 1 mm of Hg, which you can read about in the section of manometric units. In this case, 4 ata means that the water has a pressure of 3 atm (because there is 1 atm from the air above). For example, under water you have water pressure and air pressure a maverick scientist who decries the SI system may want to write the water pressure in terms of ata to include the air pressure above.
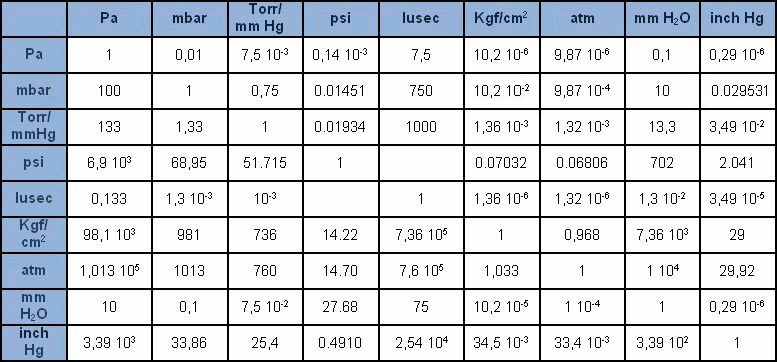

There is also a unit “total atmospheres” (ata) which is used specifically when dealing with the pressure from multiple fluids. Atmospheres are an intuitive, coarse unit of pressure. In this case I mean that I didn’t measure the air pressure, but I assumed it was basically the pressure of the room, which is more or less 1 atm.Ītmospheres might also be used in scientific fields where the reference to the real atmosphere is important, or when trying to communicate to the general public. For example, I might say that I heated my sample in dry air at 1 atm. Technically, the atm is defined as 101,325 Pa, but it was originally designated as the air pressure at sea level and 0 ✬.Ītmospheres are most commonly used when the precise air pressure is unimportant. The unit atmosphere (atm) is convenient because it is about equal to the atmospheric pressure. However, since these values are so high, usually thousands or millions of Pa, they are expressed in MPa or GPa. Materials scientists may be more likely to read air pressure in terms of Pa because we are used to using Pa to describe stress, strength, elastic modulus, and hardness Pa is exclusively used for stress, so materials scientists are quite familiar with this unit. The atmospheric pressure in pascals is 101,325 Pa, or about 100 = kPa. The standard SI unit of pressure is Pa, named after scientist Blaise Pascal. In the next section, I’ll make a big conversion chart. Let’s talk about each unit of air pressure, and give the context where it might be used. All Reasonable Units of Pressure (and quite a few unreasonable ones) Telling you that a container has 500 atm (which is 500 times the air pressure you feel right now) is probably a lot more meaningful to the average person than saying the container has 7000 psi.

Each molecule bounce imparts an imperceptible force, but if you add them all up, you get air pressure!Īlthough the Pa is the Si unit of air pressure, atmospheres (atm) are also commonly used for convenience, because 1 atm is the atmospheric pressure.
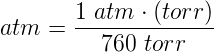
A container of gas might have waaaay more than billions of gas molecules bouncing off the walls every second. As you may know, gas particles are often modeled as tiny spheres moving really fast and bouncing off of walls. Stress is extremely important in materials science, because it describes a material any time the material experiences a force (which is always, at least because of gravity, friction, and air pressure!).Īir pressure comes from the random motion of gas particles. However, stress is still a force per area, so it’s identical to pressure. More specifically, when we are talking about solid objects, we use the term stress. Pressure allows metal boats to float, airplanes to fly, straws to suck water, balloons to hold their shape, and even describes the interaction of all materials with any force! There are many cool demonstrations you can do with pressure, such as placing a napkin in an upside-down cup underwater or boiling water with a vacuum chamber. You probably first encountered pressure when learning about the gas laws in high school chemistry. All Reasonable Units of Pressure (and quite a few unreasonable ones).


 0 kommentar(er)
0 kommentar(er)
